

For more than half a year, six people have been going about their lives with sensors implanted in blood vessels in their brains that enable them to communicate directly with their computers. The participants, who are all severely paralyzed, are taking part in a study that could change their lives and mark a turning point in brain-computer interface (BCI) technology. In 2024, they’ll find out if the tech will continue on the path to the clinic.
Until now, only about 50 humans have ever had BCIs implanted in their brains. And only a handful of those people have been able to leave the laboratory to use them in the real world, since most BCI implants involve wires protruding from the head. The new study is the largest human trial of a fully implantable, at-home BCI system.
And no, the maker of this device isn’t Elon Musk’s Neuralink. It’s a company called Synchron, and it is quietly leading the race to bring a BCI implant to market.
 Synchron’s Stentrode is inserted through the jugular vein and snaked up to a blood vessel over the motor cortex. Synchron
Synchron’s Stentrode is inserted through the jugular vein and snaked up to a blood vessel over the motor cortex. Synchron
“Synchron is the very first to commercialize the concept of BCI [implants] in a meaningful way, and they’re paving the way for the whole field,” says Nick Ramsey, a clinical neuroscientist at University Medical Center Utrecht, in the Netherlands, who is not involved in the development of Synchron’s device. It “might very well be on the market for a while before any [other] devices are competing with it,” he says.
If Synchron’s system works, it will provide an invaluable communication method to people with severe paralysis. Many potential users suffer from brain-stem stroke or degenerative diseases that have left them “locked-in”: aware of their surroundings but with no way of communicating other than blinking. With a BCI implant, they will be able to do basic computer tasks—like sending messages and accessing digital health services—without moving a muscle.
Synchron first implanted its device in four people in Australia. Then the company moved its operations to Brooklyn, N.Y., and it’s now in the middle of a U.S.-based feasibility study involving six more people. By June the company expects to submit its data to the U.S. Food and Drug Administration for review. If the results are good, the company will seek the agency’s blessing to move forward with a larger study that will determine whether this BCI gets approved for clinical use.
The Stentrode picks up neural signals from inside a blood vessel on the brain’s surface. Synchron
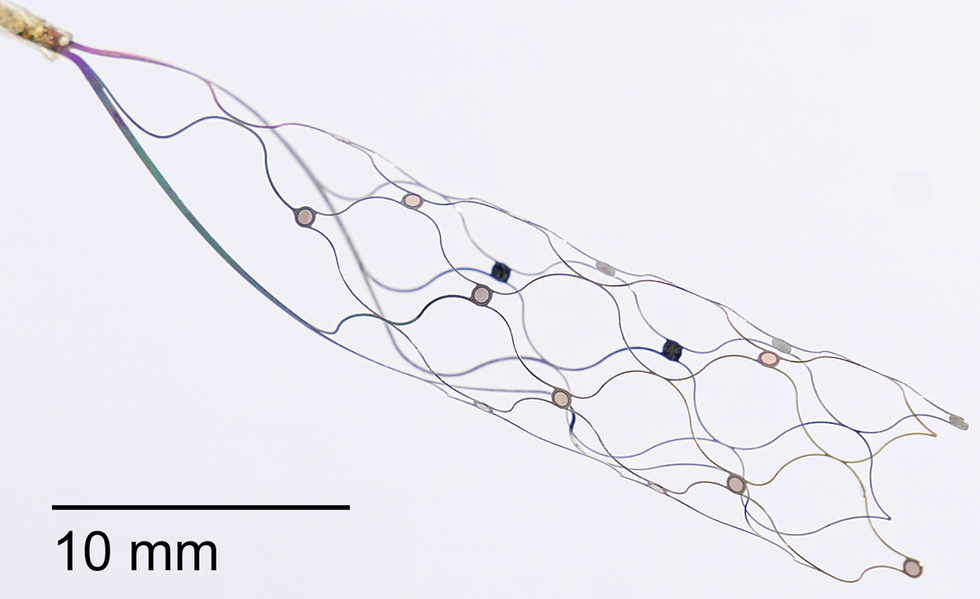
One big advantage of the Synchron device: It doesn’t require open brain surgery. Instead, it’s delivered like a stent. A 16-electrode array, trailing a lead behind it, is inserted into the jugular vein in the neck and snaked up a blood vessel near the brain’s motor cortex. When it reaches its destination, it springs out into a tubelike scaffold that fits against the inside wall of the blood vessel. There, the Stentrode records electrical activity coming from nearby brain tissue. The Stentrode is connected by the lead to a small receiver-transmitter in the chest, which wirelessly sends data to an external digital device.
To communicate with a computer, a user of Synchron’s system thinks about a specific motor movement, such as moving his or her leg. Even though the user cannot physically move, these thoughts generate electrical activity in the motor cortex that’s fairly easy to detect. The external device then translates that data into a simple computer command.
A patient with Synchron’s brain implant uses her thoughts to navigate an iPhone menu. Synchron
The only computer commands Synchron’s system can currently generate are clicks and a scrolling function. The click command can be used in conjunction with special assistive software that slowly scrolls through Web pages highlighting different areas of interest that the user can click on. Synchron is limited to these two commands because of the quality of the brain signals that can be detected from inside a blood vessel. “We are recording population-level signals from neurons, not single-unit-level signals,” says Tom Oxley, CEO of Synchron.
This setup is considerably less sophisticated than the BCIs coming from other companies and academic groups. “The current research is geared toward capturing more signals from a larger patch of cortex so that you are decoding more complex movements or speech,” says Ramsey, who is credited with testing the very first implantable BCI in a human in 2016. “To decode speech, you need at least 100 electrodes.”
Synchron’s competitors include Elon Musk’s Neuralink and rival Precision Neuroscience. Neuralink’s BCI features 1,024 electrodes distributed across 64 ultrathin threads, which must be surgically implanted by a custom robot. The company has tested it in animals and in May 2023 said that it had received a green light from the FDA to test it in humans, after the agency initially rejected the request.
Precision Neuroscience’s BCI features 1,024 electrodes on a one-square-centimeter flexible film. In 2023 the company conducted pilot studies in humans in which the film was placed temporarily on the surface of the brain while people were undergoing unrelated tumor surgery.
But the complexity of these devices and their implantation procedures may add years of clinical testing while Synchron speeds toward regulatory approval. “We are leaning into the simplicity feature of our system,” says Synchron’s Oxley. The brain signals that represent motor movement are predictable and similar in every person, he says, so the patterns can be immediately decoded; the user doesn’t have to spend weeks or months training a deep-learning algorithm to recognize the person’s unique brain patterns. “Our device works on day one,” he says.
Reference: https://ift.tt/oawngON
No comments:
Post a Comment