

Walk through half a football field’s worth of low partitions, filing cabinets, and desks. Note the curved mirrors hanging from the ceiling, the better to view the maze of engineers, technicians, and support staff of the development laboratory. Shrug when you spot the plastic taped over a few of the mirrors to obstruct that view.
Go to the heart of this labyrinth and there find M. George Craford, R&D manager for the optoelectronics division of Hewlett-Packard Co., San Jose, Calif. Sitting in his shirtsleeves at an industrial beige metal desk piled with papers, amid dented bookcases, gym bag in the corner, he does not look like anybody’s definition of a star engineer.
Appearances are deceiving.
This article was first published as “M. George Craford.” It appeared in the February 1995 issue of IEEE Spectrum. A PDF version is available on IEEE Xplore. The photographs appeared in the original print version.
“Take a look around during the next few days,” advised Nick Holonyak Jr., the John Bardeen professor of electrical and computer engineering and physics at the University of Illinois, Urbana, and the creator of the first LEDs. “Every yellow light-emitting diode you see—that’s George’s work.”
Holonyak sees Craford as an iceberg—showing a small tip but leaving an amazing breadth and depth unseen. Indeed, Craford does prove to be full of surprises—the gym bag, for example. He skips lunch for workouts in HP’s basement gym, he said, to get in shape for his next adventure, whatever that might be. His latest was climbing the Grand Teton; others have ranged from parachute jumping to whitewater canoeing.
His biggest adventure, though, has been some 30 years of research into light-emitting diodes.
The call of space
When Craford began his education for a technical career, in the 1950s, LEDs had yet to be invented. It was the adventure of outer space that called to him.
The Iowa farm boy was introduced to science by Illa Podendorf, an author of children’s science books and a family friend who kept the young Craford supplied with texts that suited his interests. He dabbled in astronomy and became a member of the American Association of Variable Star Observers. He built rockets. He performed chemistry experiments, one time, he recalls with glee, generating an explosion that cracked a window in his home laboratory. When the time came, in 1957, to pick a college and a major, he decided to pursue space science, and selected the University of Iowa, in Iowa City, because space pioneer James Van Allen was a physics professor there.
Vital statistics
Name
Magnus George Craford
Date of birth
Dec. 29, 1938
Birthplace
Sioux City, Iowa
Height
185 cm
Family
Wife, Carol; two adult sons, David and Stephen
Education
BA in physics, University of Iowa, 1961; MS and PhD in physics, University of Illinois, 1963 and 1967
First job
Weeding soybean fields
First electronics job
Analyzing satellite data from space
Patents
About 10
People most respected
Explorer and adventurer Sir Richard Burton, photographer Galen Rowell, Nobel Prize winner John Bardeen, LED pioneer Nick Holonyak Jr.
Most recent book read
The Charm School
Favorite book
Day of the Jackal
Favorite periodicals
Scientific American, Sports Illustrated, National Geographic, Business Week
Favorite music
String quartets
Favorite composers
Mozart, Beethoven
Computer
“I don’t use one”
Favorite TV show
“NYPD Blue”
Favorite food
Thai, Chinese
Favorite restaurant
Dining room at San Francisco’s Ritz Carlton Hotel
Favorite movies
Bridge on the River Kwai, Butch Cassidy and the Sundance Kid, The Lion in Winter
Leisure activity
Hiking, walking, snow skiing, bicycling, tennis, and, most recently, technical mountain climbing.
Car
Sable Wagon (a company car)
Pet peeves
“People that work for me who don’t come to me with little problems, which fester and turn into big ones.”
Organizational membership
IEEE, Society for Information Display
Favorite awards
National Academy of Engineering, IEEE Fellow, IEEE Morris N. Liebmann Memorial Award; but “everything you do is a team thing, so I have mixed feelings about awards.”
As the space race heated up, Craford’s interest in space science waned, in spite of a summer job analyzing data returned from the first satellites. He had learned a bit about semiconductors, an emerging field, and Van Allen pointed him toward the solid-state physics program at the University of Illinois, where Craford studied first for a master’s degree, then a PhD.
The glowing Dewar
For his doctoral thesis, Craford began investigating tunneling effects in Josephson junctions. He had invested several years in that research when Holonyak, a pioneer in visible lasers and light-emitting diodes, left his position at General Electric Co. and joined the Illinois faculty. Craford met him at a seminar, where Holonyak was explaining his work in LEDs. Recalled Craford: “He had a little LED—just a red speck—and he plunged it into a Dewar of liquid nitrogen, and it lit up the whole flask with a bright red light.”
Entranced, Craford immediately spoke to his thesis adviser about switching, a fairly unusual proposal, since it involved dropping years of work. “My thesis adviser was good about it; he had been spending less time around the lab lately, and Holonyak was building up a group, so he was willing to take me on.”
Craford believes he persuaded the laser pioneer to accept him, the senior man recalls things differently.
Craford’s adviser “was running for U.S. Congress,” Holonyak said, “and he told me, ‘I’ve got this good student, but I’m busy with politics, and everything we do someone publishes ahead of me. I can’t take good care of him. I’d like you to pick him up.”’
However it happened, Craford’s career path was finally set—and the lure of the glowing red Dewar never dimmed.
Holonyak was growing gallium arsenide phosphide and using it successfully to get bright LEDs and lasers. He assigned his new advisee the job of borrowing some high-pressure equipment for experiments with the material. After finding a professor with a pressure chamber he was willing to lend, Craford set up work in the basement of the materials research building. He would carry GaAsP samples from the lab to the materials research basement, cool them in liquid nitrogen, increase the pressure to study the variation of resistivity, and see unexpected effects.
“Just cooling some samples would cause the resistance to go up several times. But add pressure, and they would go up several orders of magnitude,” Craford said. “We couldn’t figure out why.”
Eventually, Craford and a co-worker, Greg Stillman, determined that variations in resistance were related not only to pressure but also to light shining on the samples. “When you cooled a sample and then shone the light on it, the resistance went down—way down—and stayed that way for hours or days as long as the sample was kept at low temperature, an effect called persistent photoconductivity.” Further research showed that it occurred in samples doped with sulfur but not tellurium. Craford and Stillman each had enough material for a thesis and for a paper published in the Physical Review.
The phenomenon seemed to have little practical use, and Craford put it out of his mind, until several years later when researchers at Bell Laboratories found it in gallium aluminum arsenide. “Bell Labs called it the DX Center, which was catchy, studied it intensively, and over time, many papers have been published on it by various groups,” Craford said. Holonyak’s group’s contribution was largely forgotten.
“He doesn’t promote himself,” Holonyak said of Craford, “and sometimes this troubles me about George; I’d like to get him to be more forward about the fact that he has done something.”
Move to Monsanto
After receiving his PhD, Craford had several job offers. The most interesting were from Bell Laboratories and the Monsanto Co. Both were working on LEDs, but Monsanto researchers were focusing on gallium arsenide phosphide, Bell researchers on gallium phosphide. Monsanto’s research operation was less well known than Bell Labs’ and taking the Monsanto job seemed to be a bit of a risk. But Craford, like his hero—adventurer Richard Burton, who spent years seeking the source of the Nile—has little resistance to choosing the less well-trodden path.
Besides, “Gallium phosphide just didn’t seem right,” said Craford, “but who knew?”
In his early days at Monsanto, Craford experimented with both lasers and LEDs. He focused on LEDs full time when it became clear that the defects he and his group were encountering in growing GaAsP on GaAs substrates would not permit fabrication of competitive lasers.
[He] didn’t toot his own horn. “When George [Craford] published the work, he put the names of the guys he had growing crystals and putting the things together ahead of his name.”
—Nick Holonyak
The breakthrough that allowed Craford and his team to go beyond Holonyak’s red LEDs to create very bright orange, yellow, and green LEDs was prompted, ironically, by Bell Labs. A Bell researcher who gave a seminar at Monsanto mentioned the use of nitrogen doping to make indirect semiconductors act more like direct ones. Direct semiconductors are usually better than indirect for LEDs, Craford explained, but the indirect type still has to be used because of band gaps wide enough to give off light in the green, yellow, and orange part of the spectrum. The Bell researcher indicated that the labs had had considerable success with Zn-O doping of gallium phosphide and some success with nitrogen doping of gallium phosphide. Bell Labs, however, had published early experimental work suggesting that nitrogen did not improve GaAsP LEDs.
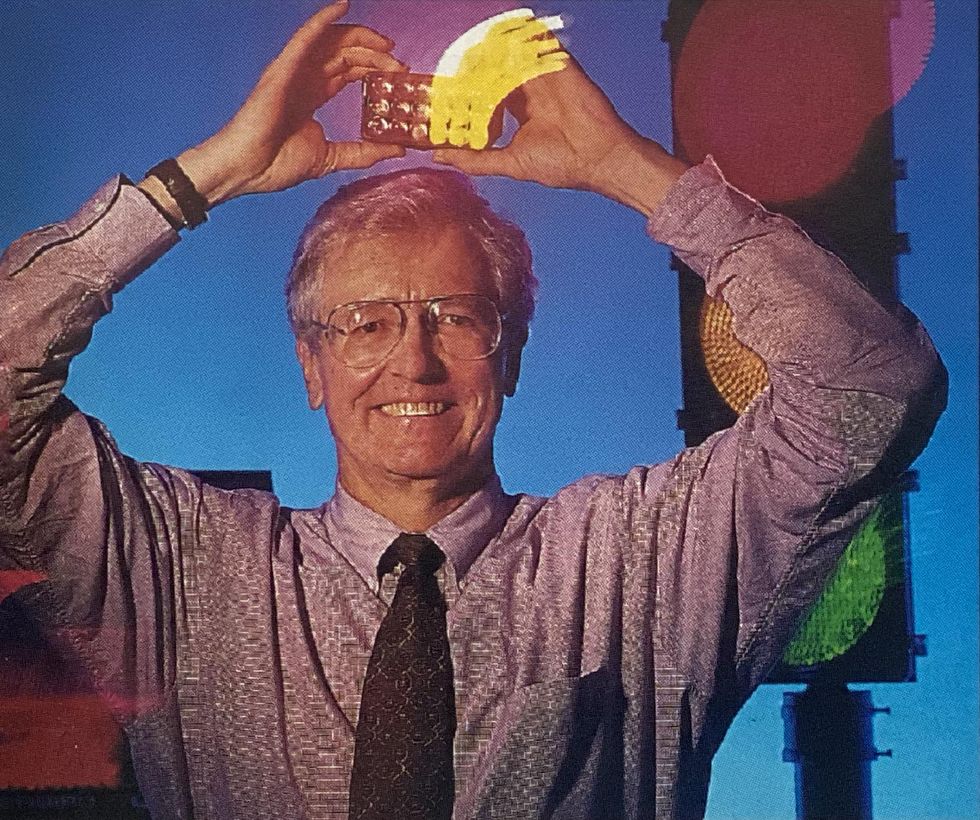
Nonetheless, Craford believed in the promise of nitrogen doping rather than the published results. “We decided that we could grow better crystal and the experiment would work for us,” he said.
A small team of people at Monsanto did make it work. Today, some 25 years later, these nitrogen-doped GaAsP LEDs still form a significant proportion—some 5-10 billion—of the 20-30 billion LEDs sold annually in the world today.
“The initial reaction was, ‘Wow, that’s great, but our customers are very happy with red LEDs. Who needs other colors?’”
—George Craford
Again, Holonyak complains, Craford didn’t toot his own horn. “When George published the work, he put the names of the guys he had growing crystals and putting the things together ahead of his name.”
His peers, however, have recognized Craford as the creative force behind yellow LEDs, and he was recently made a member of the National Academy of Engineering to honor this work.
Craford recalls that the new palette of LED colors took some time to catch on. “The initial reaction,” he said, “was, ‘Wow, that’s great, but our customers are very happy with red LEDs. Who needs other colors?’”
Westward ho!
After the LED work was published, a Monsanto reorganization bumped Craford up from the lab bench to manager of advanced technology. One of his first tasks was to select researchers to be laid off. He recalls this as one of the toughest jobs of his life, but subsequently found that he liked management. “You have more variety; you have more things that you are semi-competent in, though you pay the price of becoming a lot less competent in any one thing,” he told IEEE Spectrum.
Soon, in 1974, he was bumped up again to technology director, and moved from Monsanto’s corporate headquarters in St. Louis to its electronics division headquarters in Palo Alto, Calif. Craford was responsible for research groups developing technology for three divisions in Palo Alto, St. Louis, and St. Peters, Mo. One dealt with compound semiconductors, another with LEDs, and the third with silicon materials. He held the post until 1979.
Even as a manager, he remained a “scientist to the teeth,” said David Russell, Monsanto’s director of marketing during Craford’s tenure as technology director. “He is a pure intellectual scientist to a fault for an old peddler like me.”
Though always the scientist, Craford also has a reputation for relating well to people. “George is able to express complicated technical issues in a way that all of us can understand,” said James Leising, product development manager for HP’s optoelectronics division.
Leising recalled that when he was production engineering manager, a position that occasionally put him in conflict with the research group, “George and I were always able to work out the conflicts and walk away friends. That wasn’t always the case with others in his position.” One time in particular, Leising recalled, Craford convinced the production group of the need for precise control of its processes by graphically demonstrating the intricacies of the way semiconductor crystals fit upon one another.
As an executive, Craford takes credit for no individual achievements at Monsanto during that time, but said, “I was proud of the fact that, somehow, we managed to be worldwide competitors in all our businesses.” Even so, Monsanto decided to sell off its optoelectronics business and offered Craford a job back in St. Louis, where he would have been in charge of research and development in the company’s silicon business.
Craford thought about this offer long and hard. He liked Monsanto; he had a challenging and important job, complete with a big office, oak furniture, a private conference room, and a full-time administrative assistant. But moving back to St. Louis would end his romance with those tiny semiconductor lights that could make a Dewar glow, and when the time came, he just couldn’t do it.
He did the Silicon Valley walk: across the street to the nearest competitor, in this case, Hewlett-Packard Co.
Instead, he did the Silicon Valley walk: across the street to the nearest competitor, in this case, Hewlett-Packard Co. The only job it could find that would let him work with LEDs was a big step down from technology director—a position as R&D section manager, directing fewer than 20 people. This meant a cut in salary and perks, but Craford took it.
The culture was different, to say the least. No more fancy office and private conference room; at HP Craford gets only “a cubby, a tin desk, and a tin chair.”
And, he told Spectrum, “I love it.”
He found the HP culture to be less political than Monsanto’s, and believes that the lack of closed offices promotes collaboration. At HP, he interacts more with engineers, and there is a greater sense that the whole group is pulling together. It is more open and communicative—it has to be, with most engineers’ desks merely 1.5 meters apart. “I like the whole style of the place,” he declared.
Now he has moved up, to R&D manager of HP’s optoelectronics division, with a larger group of engineers under him. (He still has the cubby and metal desk, however.)
As a manager, Craford sees his role as building teams, and judging which kinds of projects are worth focusing on. “I do a reasonably good job of staying on the path between being too conservative and too blue sky,” he told Spectrum. “It would be a bad thing for an R&D manager to say that every project we’ve done has been successful, because then you’re not taking enough chances; however, we do have to generate enough income for the group on what we sell to stay profitable.”
Said Fred Kish, HP R&D project manager under Craford: “We have embarked upon some new areas of research that, to some people, may have been questionable risks, but George was willing to try.”
Craford walks that path between conservatism and risk in his personal life as well, although some people might not believe it, given his penchant for adventurous sports: skydiving, whitewater canoeing, marathon running, and rock climbing. These are measured risks, according to Craford: ‘‘The Grand Teton is a serious mountain, but my son and I took a rock-climbing course, and we went up with a guy who is an expert, so it seemed like a manageable risk.”
Holonyak recalls an occasion when a piece of crystal officially confined to the Monsanto laboratory was handed to him by Craford on the grounds that an experiment Holonyak was attempting was important. Craford “could have gotten fired for that, but he was willing to gamble.”
“I hope to see the day when LEDs will illuminate not just a Dewar but a room.”
—George Craford
Craford is also known as being an irrepressible asker of questions.
“His methods of asking questions and looking at problems brings people in the group to a higher level of thinking, reasoning, and problem-solving,’’ Kish said.
Holonyak described Craford as “the only man I can tolerate asking me question after question, because he is really trying to understand.”
Craford’s group at HP has done work on a variety of materials over the past 15 years, including gallium aluminum arsenide for high-brightness red LEDs and, more recently, aluminum gallium indium phosphide for high-brightness orange and yellow LEDs.
The latest generation of LEDs, Craford said, could replace incandescent lights in many applications. One use is for exterior lighting on automobiles, where the long life and small size of LEDs permit car designers to combine lower assembly costs with more unusual styling. Traffic signals and large-area display signs are other emerging applications. He is proud that his group’s work has enabled HP to compete with Japanese LED manufacturers and hold its place as one of the largest sellers of visible-light LEDs in the world.
Craford has not stopped loving the magic of LEDs. “Seeing them out and used continues to be fun,” he told Spectrum. “When I went to Japan and saw the LEDs on the Shinkansen [high-speed train), that was a thrill.”
He expects LEDs to go on challenging other forms of lighting and said, “I still hope to see the day when LEDs will illuminate not just a Dewar but a room.”
Editor’s note: George Craford is currently a fellow at Philips LumiLEDs. He got his wish and then some.
Reference: https://ift.tt/EB9aWsF


 Oleg Boyarintsev is here, back at work after detention and questioning by Ukrainian counterintelligence agents. Energoatom
Oleg Boyarintsev is here, back at work after detention and questioning by Ukrainian counterintelligence agents. Energoatom Under CEO Petro Kotin, Energoatom has faced repeated accusations of corruption and sliding back toward Russian influence. Ukrinform/Alamy
Under CEO Petro Kotin, Energoatom has faced repeated accusations of corruption and sliding back toward Russian influence. Ukrinform/Alamy Alleged Russian spy Andriy Derkach [right] meeting in Kiev with former President Trump’s lawyer Rudy Giuliani in 2019.Andriy Derkach
Alleged Russian spy Andriy Derkach [right] meeting in Kiev with former President Trump’s lawyer Rudy Giuliani in 2019.Andriy Derkach




























 Toyota Research Institute
Toyota Research Institute A robot for your home may not look much like this research platform, but it’s how TRI is learning to make home robots that are useful and safe. Tidying and cleaning are physically repetitive tasks that are ideal for home robots, but still a challenge since every home is different, and every person expects their home to be organized and cleaned differently.Toyota Research Institute
A robot for your home may not look much like this research platform, but it’s how TRI is learning to make home robots that are useful and safe. Tidying and cleaning are physically repetitive tasks that are ideal for home robots, but still a challenge since every home is different, and every person expects their home to be organized and cleaned differently.Toyota Research Institute A robot for your home may not look much like this research platform, but it’s how TRI is learning to make home robots that are useful and safe. Perceiving and grasping transparent objects like drinking glasses is a particularly difficult task.Toyota Research Institute
A robot for your home may not look much like this research platform, but it’s how TRI is learning to make home robots that are useful and safe. Perceiving and grasping transparent objects like drinking glasses is a particularly difficult task.Toyota Research Institute These bubble grippers are soft to the touch, making them safe for humans to interact with, but they also include the necessary sensing to be able to grasp and identify a wide variety of objects.Toyota Research Institute
These bubble grippers are soft to the touch, making them safe for humans to interact with, but they also include the necessary sensing to be able to grasp and identify a wide variety of objects.Toyota Research Institute







